Conflicts of Interest: The U.S. Food and Drug Administration
My new article with the Canadian Covid Care Alliance

Hello, friends!
The Canadian Covid Care Alliance has just published a trio of articles authored by me and two very good friends of mine, Deanna McLeod and Matthew Evans-Cockle. As of yesterday, we are officially known as the CCCA Conflict of Interest Task Force!
We’ve been working as a trio for well over a year now, focused on researching and analyzing conflicts of interest throughout the national and international response to the declared COVID-19 pandemic. This first set of articles is just the beginning of our output, with further pieces to come examining other agencies in the United States and beyond.
That’s not all: as we were in the final stages of editing these articles, we were given the opportunity to submit them for inclusion in the Canadian Covid Care Alliance’s upcoming book, titled Down the COVID-19 Rabbit Hole: Independent Scientists and Physicians Unmask the Pandemic, scheduled for publication on August 6, 2024 by Skyhorse Publishing and Children’s Health Defense.

Drs. Steven Pelech and Christopher Shaw did a fantastic job editing together over a dozen chapters from some two dozen contributors, and I can’t wait to see the end result. I am positively thrilled, and I hope you are just as excited! It’s a ways off, but you can preorder the book on Amazon.
In the meantime, without further ado, I’d like to share with you (in full) the second article in this new trio, titled “Conflicts of Interest: The U.S. Food and Drug Administration”. The full PDF is available for download at the end of this post. Enjoy!
The Food and Drug Administration (FDA) is an agency of the United States government sitting within the United States Department of Health and Human Services (HHS). It is responsible for regulating the “safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices” and “ensuring the safety of [the United States’] food supply, cosmetics, and products that emit radiation.”1
The FDA was officially founded in 1906 when U.S. President Theodore Roosevelt signed the Pure Food and Drugs Act into law. The bill is described as the “culmination of about 100 bills over a quarter-century that aimed to rein in long-standing, serious abuses in the consumer product marketplace.”2 A primary motivation behind the creation of the FDA was to “prohibit the adulteration and misbranding of food and drugs.”3 Today, it also “regulates clinical investigations of products under its jurisdiction, such as drugs, biological products, and medical devices.”4 The FDA does this work by receiving a dossier of evidence submitted by companies in these regulated industries, and evaluating whether the product meets established standards for safety and efficacy. If the agency decides these standards have been met, the product is approved for distribution.
The current Commissioner of the FDA is Dr. Robert M. Califf, who was promoted to the position on February 17, 2022.5
Budget and Funding
As a federal agency, the FDA is allocated public funding on an annual basis by the United States Congress. In 1992, however, Congress enacted the Prescription Drug User Fee Act (PDUFA) which made it legal for the FDA to receive “user fees” from pharmaceutical companies for each new drug submitted for review.6 This allowed the agency to dramatically increase the rate at which new drugs were approved by expanding their staff, leading to record-breaking numbers of new drug approvals by 1996. To expedite the process still further, the FDA introduced a new “priority application” service that allowed drugs to be approved in half the time that “standard applications” previously required.7 In 1993, 27% of the portion of the FDA’s budget specifically earmarked for reviewing new drugs was paid for by the pharmaceutical industry. This figure increased to over 50% by 2006,8 and 75% by 2017.9 From 2019-2021, the FDA’s overall budget increased from $5.7 billion to $6.1 billion, of which 45-46% came from the pharmaceutical industry through the above-described “user fees.”101112
At its inception, the Food and Drug Administration was funded almost exclusively by the public it was meant to protect. Over the course of the past century, however, the FDA has become increasingly funded by the private industries it was meant to regulate. It is hard to imagine how this organization can maintain the unbiased objectivity necessary to its regulatory function if its ability to operate is dependent on industry funding. On the one hand, if the FDA's dependence upon pharmaceutical industry funding did not compromise but actually reinforced the rigorous application of a high standard of safety testing, then the industry subsidized bolstering of FDA funding would represent a tremendous win for both industry and the public. On the other hand, if this funding dependence had the regrettable effect of speeding up the drug approval process while also lowering safety standards, then this would constitute a very serious threat to public welfare.
Leading into the COVID-19 crisis, just under half of the entire operating budget of the FDA was paid for by the companies that the agency was mandated to regulate. Indeed, a significant portion of 2021’s budgetary increase came from a $2.8 million application fee, paid by Pfizer to the FDA in May 2021, for an expedited review of their submission for Emergency Use Authorization of their COVID-19 mRNA genetic vaccine product, BNT162b2.13
Speeding up the development of life-saving new drugs has the potential to do great good, particularly in the case of new technological advances. At the same time, if this were to result in cutting corners on necessary protections and on crucial stages of the regulatory process, then this would go against the primary aim and function of the FDA and puts consumers at risk. In the case of the Pfizer-BioNTech COVID-19 genetic vaccine (officially called BNT162b2), the exchange of fees for the expedited approval of a novel gene therapy for use in healthy Americans is certainly concerning – particularly given that less than 2 months of safety data was available for the FDA to review at the time of its conditional approval.14 The Pfizer-BioNTech product is, by definition, a gene therapy, a category of product that can require up to 15 years of testing to satisfy the FDA’s requirements.15 However, by allowing the mRNA products to be reviewed as a vaccine candidate, it was subject to much lower safety standards.
User fees are not the only way in which the FDA is at risk of financial influence by the pharmaceutical industry. There are, additionally, a number of so-called “non-profit” organizations, such as the Reagan-Udall Foundation for the FDA and the Alliance for a Stronger FDA, whose primary focus is “improving” the processes of the FDA, while also lobbying the United States government to further increase the agency’s yearly budget.
Reagan-Udall Foundation for the FDA
The Reagan-Udall Foundation for the Food and Drug Administration,
often referred to as the FDA Foundation, is a non-profit organization established by the U.S. Congress in 2007.

Officially, this foundation operates independently of the FDA and is intended “to help support and promote FDA's regulatory science priorities.”16 It fulfills this mission by collaborating with pharmaceutical and biotechnology companies on research programs, evidence generation and data gathering, as well as by analyzing and creating reports on the operations of the FDA.17 As there is clear continuity and even fundamental interdependence between the FDA and the Reagan-Udall Foundation, it might be more accurate to say that the Foundation functions as an arm of the FDA. Unlike the main body of the FDA, this Reagan-Udall arm is free to pursue industry partnerships as it is not restricted in its operations by the latter’s original mandate and regulatory limits.
Funding
Funding for the Reagan-Udall Foundation is made up of an annual $1.25 million budget provided by the FDA, with the remainder coming from grants, donations, research contracts, fundraising events and investment income. The Reagan-Udall Foundation’s annual budget nearly doubled over the COVID-19 period. In 2019, the Foundation’s reported revenue was just over $2.7 million.18 This jumped to $4.9 million in 2020,19 and to $5.5 million in 2021.20 Thus, as of 2021, 77.3% of the Foundation’s grants came from industry or private philanthropic entities that have strong ties to industry.21
Among the Reagan-Udall Foundation’s financial supporters are companies and organizations that have developed and profited handsomely from therapeutics and medical interventions deployed in response to COVID-19 including Pfizer, AstraZeneca, Janssen (along with parent company Johnson & Johnson), and Flagship Pioneering, the venture capital firm that launched Moderna.22
Even more pharmaceutical industry-specific funding comes from industry associations, including the Biotechnology Innovation Organization (BIO), Consumer Healthcare Products Association (CHPA) and Pharmaceutical Research and Manufacturers of America (PhRMA). These organizations serve as lobbying groups representing the major pharmaceutical companies just mentioned, as well as a host of other companies involved in the research, development, and marketing of pharmaceutical products.232425
Finally, the Bill & Melinda Gates Foundation and the Rockefeller Foundation are both substantial donors to the Reagan-Udall Foundation. These private and incredibly influential philanthropic foundations are themselves extensions of powerful corporate entities that have developed substantial portfolios of pharmaceutical investments both in the private and public sector, and have thus stood to profit heavily from the FDA’s favourable recommendations of patented pharmaceutical products (as opposed to non-pharmaceutical or generic products) for the treatment of COVID-19.
These non-governmental organizations are recognized for pioneering and then institutionalizing global “philanthrocapitalism.”26 Successful philanthrocapitalist foundations make use of ostensibly charitable donations to further corporate ventures. Their stated intentions are always along the lines of advancing global health, welfare, and other humanitarian initiatives, but the true result of their generosity is identified in their increased profit margins. Particularly notable in this case are the Bill & Melinda Gates Foundation’s investments in Pfizer,27 Moderna,28 Janssen,29 AstraZeneca,30 and BioNTech,31 whose COVID-19 vaccine products were the first to be authorized for use in Canada and the United States. The Bill & Melinda Gates Foundation is also a primary funder of the world’s largest non-profit organizations focused on vaccines, including Gavi, the Vaccine Alliance,32 the Coalition for Epidemic Preparedness Innovations (CEPI),33 and the Global Fund to Fight AIDS, Tuberculosis and Malaria.34 These organizations are tasked with funding and delivering vaccines across countries around the world, developed by companies in which the Gates Foundation has a financial stake.35 Therefore, the Gates Foundation represents both sides of the decision making process—an arrangement that Bill Gates himself claimed in 2019 has led to “a 20-to-1 return” on investment over the years.36 As such, the Bill & Melinda Gates Foundation’s funding of the Reagan-Udall Foundation presents the concerning possibility that it may also be able to incentivize favourable regulatory outcomes for the vaccines in which it has invested.
The Reagan-Udall Foundation identifies itself as a public-private partnership. In this supporting role, it becomes possible for the Foundation to influence FDA activities by a coordinated network of interested parties, including major pharmaceutical companies, their lobbying associations, global philanthrocapitalist foundations and other non-governmental organizations. The foundation’s enabling of corporate collaboration allows private interests to influence the regulatory environment in which they pursue their corporate agendas. In other words, the legally distinct Food and Drug Administration and the Reagan-Udall Foundation could be seen as operating as two branches of a functionally single FDA enterprise. Grafting the Reagan-Udall Foundation to the Food and Drug Administration appears to risk circumventing the vital restrictions placed upon the regulatory agency in its original mandate.
COVID-19 Vaccine Confidence Project
As previously described, the FDA is not intended to promote medical products. The agency’s intended role and mandate is strictly regulatory—to ensure that only safe and effective products reach the market; these same restrictions, however, do not apply to the Reagan-Udall Foundation.
In the first few months of the COVID-19 crisis in early 2020, the National Science Foundation, an agency of the United States government, funded an initiative at the Johns Hopkins Center for Health Security (JHCFS) called the Working Group on Readying Populations for COVID-19 Vaccine.37 The Center for Health Security is an institution within Johns Hopkins University that has been involved in pandemic preparedness and “biosecurity” since its inception in 1998.38 It has hosted a number of tabletop exercises simulating the outbreak of viral pandemics, among which are simulations of global coronavirus pandemics such as Event 201 (2019)39 and the earlier SPARS (2017).40 Notably, the membership of the Center for Health Security’s Working Group included representatives of In-Q-Tel, the venture capital branch of the U.S. Central Intelligence Agency.4142 The JHCHS Working Group proposed a “social and behavioral research agenda to facilitate COVID-19 vaccine uptake.”43 In its report, the Working Group makes specific recommendations for the FDA and other institutions (emphasis added):
“In advance of a SARS-CoV-2 vaccine rollout, federal health agencies should develop a coordinated national strategy to promote vaccination, employing human-centered design to develop interventions that help a broad network of champions communicate effectively with the public about risks, benefits, allocation and targeting, and availability. The National Vaccine Program Office at the US Department of Health and Human Services (HHS) can coordinate the CDC, the FDA, and the NIH in developing a COVID-19 vaccine promotion campaign. Specifically, the Office of Minority Health at HHS, the Office of Minority Health and Health Equity at CDC, the Indian Health Service, and the Office of Minority Health and Health Equity at FDA should be involved. To assure the effectiveness of all SARS-CoV-2 vaccine communication (Recommendation #4), serial (ie, repeated) surveys of the public, including subgroups, as well as targeted qualitative research among essential, hesitant, and underserved groups will be necessary to know what people are thinking, how this evolves over time, and if communication messages need to be adapted. While the federal health agencies may lead this national effort, it will be critical to enlist non government actors such as employers, human rights groups, minority interest groups, and other stakeholders in whom diverse segments of the US public may place more trust.”
As a direct result of these recommendations, the FDA recruited the Reagan-Udall Foundation to run a $209,094 “COVID-19 vaccine confidence project” in the fall of 2020.44 Specifically, the project involved identifying causes of so-called “vaccine hesitancy” in various target populations and developing “a set of messages that responded to their concerns.” These messages were then “delivered to FDA for use in their messaging.”
This project—to develop preemptive messaging that encourages vaccine confidence—represents a significant investment of resources from the federal government to maximize uptake of the novel COVID-19 vaccine candidates before they had been proven safe and effective. Furthermore, the phrasing in the JHCHS Working Group recommendations makes clear that these “serial... surveys of the public” were not to be carried out as good faith consultation to properly identify and respond to the public interest, but rather as a strategically designed surveillance campaign for the purpose of ensuring compliance. This results in the overriding of the process of informed consent.
Notably, the JHCHS and Johns Hopkins University have also received substantial funding over the years from the Bill & Melinda Gates Foundation and Rockefeller Foundation.454647 Its sister organizations, the Johns Hopkins Center for Immunization Research and Johns Hopkins Center for American Indian Health, also participated as sites for the Phase III clinical trial for the Pfizer-BioNTech COVID-19 vaccine product candidate.48 The Center for Immunization Research additionally served as a trial site for the AstraZeneca adenovirus vector vaccine product.49
Furthermore, in April 2019, the JHCHS published a report titled Vaccine Platforms: State of the Field and Looming Challenges, generated following consultations with Moderna and other companies developing mRNA vaccine products.50 It described the way in which regulatory agencies like the FDA could, in the near future, expedite the approval process of novel vaccine “platforms”—exactly as was done with the COVID-19 mRNA-based vaccines and their subsequent “bivalent” replacements. The report even forecasts that “an mRNA-based vaccine platform technique appears particularly promising” for pandemic preparedness and that “their proliferation appears imminent,” dependent on “clinical safety and efficacy trials and regulatory agency compliance.”
The FDA has a fiduciary responsibility to objectively evaluate the safety and efficacy of the COVID-19 genetic products. Instead of acquitting itself of this responsibility, however, the FDA, under the direction of the National Vaccine Program Office at the US Department of Health and Human Services (HHS) and under advisement of the JHCHS working group, engaged in pre-marketing activities for these same products, thereby demonstrating its lack of objectivity and independence. It bears repeating: the FDA explicitly prioritized the implementation of a pre-marketing campaign for experimental products they had not yet evaluated.
COVID-19 Evidence Accelerator
Another noteworthy project within the Reagan-Udall Foundation’s purview was the COVID-19 Evidence Accelerator.51 This initiative was undertaken in collaboration with a lobbying organization called the Friends of Cancer Research, and had a budget of $1,219,555 across 2020 and 2021.
One of the studies produced by the COVID-19 Evidence Accelerator was a meta-analysis evaluating the role of hydroxychloroquine and an antibiotic called azithromycin, both alone and in combination, as treatment for COVID-19.52 This particular early treatment combination had already been used extensively by front-line physicians to successfully treat the early phase of COVID-19.535455
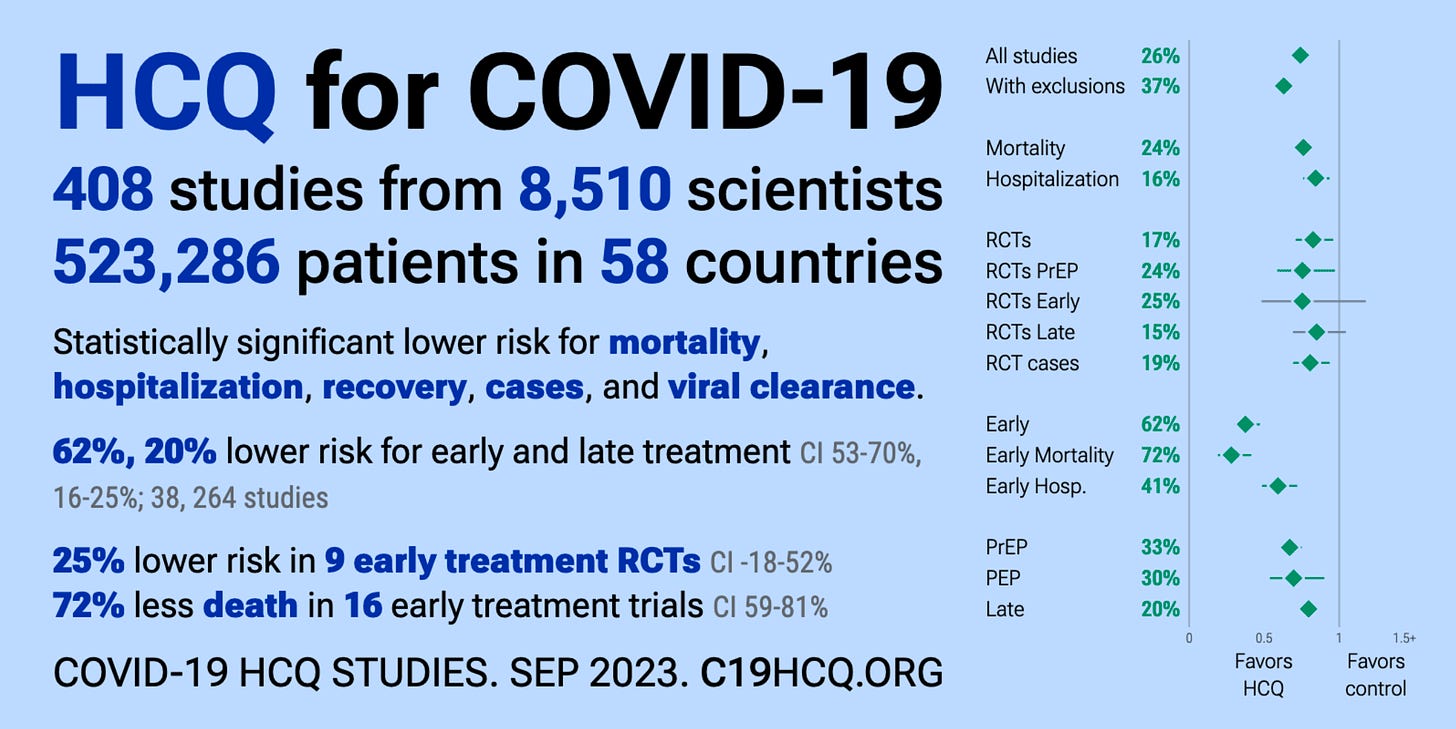
A favourable study outcome would mean that this inexpensive and readily available regimen could be immediately administered to millions of Americans with the potential to save millions of lives. Considering the established use of hydroxychloroquine and azithromycin as an early treatment protocol, one might reasonably expect the meta-analysis to examine their efficacy in the early phase of illness. Curiously, however, the study by the COVID-19 Evidence Accelerator examined the use of these treatments in late stage disease. Not surprisingly, the combination showed little to no benefit in treating late-stage COVID-19. Based on the results of this study, the authors concluded that this long-standing safe, effective and inexpensive regimen had little benefit at any stage of treatment of COVID-19, opening up the opportunity for multiple pharmaceutical companies to seek Emergency Use Authorization for a range of branded antivirals and antibody therapies.
One can’t help but wonder whether the pharmaceutical industry’s subsidization of the Reagan-Udall Foundation, as well as the competing financial ties among the authors to the very companies seeking Emergency Use Authorizations for their novel and expensive products (such as Gilead,56 Merck,57 Amgen,58 and Roche59), might have negatively influenced the design and interpretation of this study. Particularly notable is that two of the study’s authors were paid employees of Gilead Sciences, whose experimental antiviral remdesivir was simultaneously being considered for Emergency Use Authorization by the FDA.60
Alliance for a Stronger FDA
In addition to direct pharmaceutical contributions, the FDA’s
government funding is further bolstered through the efforts of a lobbying group called the Alliance for a Stronger FDA.

The Alliance is an industry association that represents the voice of the pharmaceutical industry, both to the government and general public. As such, its priority is in minimizing cost of development and maximizing the return on investment of their members’ products. One way this can be done is by reducing the amount of time pharmaceutical products take to get to market.
One of the Alliance’s primary activities is lobbying the U.S. Congress to increase the annual budget for the FDA. Between 2019-2021, the Alliance spent some $855,000 on its lobbying activities.61
The Alliance for a Stronger FDA’s membership consists of pharmaceutical companies and other industry coalitions. One of its founding companies was AstraZeneca (developer of a COVID-19 vaccine),62 and current members include both Johnson & Johnson and Pfizer.63
Non-profit organizations and industry associations that are also members of the Alliance include the Advanced Medical Technology Association (AdvaMed), Alliance for Aging Research, Alliance for Patient Access, Alliance for Regenerative Medicine, Biotechnology Innovation Organization (BIO), Consumer Healthcare Products Association (CHPA), International Society for Stem Cell Research, Personalized Medicine Coalition (PMC), Pharma and Biopharma Outsourcing Association, Pharmaceutical Research and Manufacturers of America (PhRMA), and Research!America. These purpose-built organizations and associations directly engage both government and the general public in order to promote the interests of their corporate membership.
The manner in which these associations fit together and coordinate their activities is remarkable both for its intricacy and effectiveness. To illustrate, Pharmaceutical Research and Manufacturers of America (PhRMA) is a member of the Personalized Medicine Coalition,64 which is itself a member of the Alliance for a Stronger FDA. PhRMA is, as its title suggests, a collective lobbying group comprising many of the world’s most powerful pharmaceutical corporations.65 The Personalized Medicine Coalition is an association—drawing together a network of pharmaceutical companies, healthcare facilities, and providers—that promotes commercial diagnostic tests and the medical treatments that can be prescribed on the basis of these tests. The diagnostic equipment and medical treatments in question are developed, marketed, and administered by the members of PMC. It may be somewhat counterintuitive for the uninitiated, but it is essential to recognize that PhRMA, PMC, and the Alliance For a Stronger FDA work in different ways to promote the interests of the same pharmaceutical corporations. Behind the not-for-profit facade of these non-profit lobbying groups, we see a network of powerful pharmaceutical interests working to orchestrate preferential outcomes, while benefiting from the general public’s lack of corporate literacy to conceal the extent and magnitude of their influence.
In summary
The FDA was designed to be an independent regulatory body. In recent decades, however, it has developed extensive ties to industry, both directly through user fees, and indirectly through its symbiotic relationships with non-profit organizations and lobbying groups. The financial dependence it has developed upon the pharmaceutical industry poses a very real threat to its ability to maintain effective regulatory independence. At the start of the COVID-19 crisis, essentially half of the FDA’s budget was paid for by the pharmaceutical industry, making the agency beholden to the very corporations it is meant to regulate.
Before any data was available on the safety or efficacy of the COVID-19 vaccines, the FDA worked with the Reagan-Udall Foundation to develop a pre-marketing campaign to increase confidence in and overall uptake of the forthcoming COVID-19 genetic vaccines.
The Reagan-Udall Foundation for the FDA—a Congressionally-created non-profit organization established to support the operations and improvement of the FDA—is heavily funded by pharmaceutical companies, as well as by associations that represent pharmaceutical interests. A key project led by the Foundation, the COVID-19 Evidence Accelerator, sponsored a deeply flawed meta-analysis that had the effect of undermining claims—based upon abundant real-world evidence from treating physicians—for the safety and efficacy of hydroxychloroquine and azithromycin when used in combination to treat the early phase of COVID-19. The apparent invalidation of this treatment option ultimately enabled the Foundation’s pharmaceutical funders to successfully pursue Emergency Use Authorizations for their more expensive experimental products.
The Alliance for a Stronger FDA is an association that represents the voice of the pharmaceutical industry to government and the public. Its membership consists of pharmaceutical companies as well as other organizations and coalitions that are not mandated to serve the public interest, but are, on the contrary, purpose-built to promote private corporate interests.
As thoroughly demonstrated above, the FDA is financially dependent on pharmaceutical companies. Through the Reagan-Udall Foundation, it engaged in pre-marketing activities to promote the COVID-19 vaccines before these products were deemed safe, and played a role in undermining an established treatment protocol that would have blocked access to EUA by its pharmaceutical industry sponsors. These facts call into question the FDA’s ability to fulfill its regulatory mandate and objectively evaluate the various COVID-19-related pharmaceutical products.
What we do. (2018, March 28). Food and Drug Administration. http://archive.today/2023.02.07-154218/https://www.fda.gov/about-fda/what-we-do
When and why was FDA formed? (2018, March 28). Food and Drug Administration. http://archive.today/2023.09.07-201200/https://www.fda.gov/about-fda/fda-basics/when-and-why-was-fda-formed
Swann, J. P. (2019, March 15). FDA’s origin. Food and Drug Administration. https://web.archive.org/web/20230907201447/https://www.fda.gov/about-fda/changes-science-law-and-regulatory-authorities/fdas-origin
Office for Human Research Protections (OHRP). (2016, March 18). Food & Drug Administration. United States Department of Health and Human Services. http://archive.today/2020.08.11-183732/https://www.hhs.gov/ohrp/regulations-and-policy/regulations/fda/index.html
FDA Commissioner. (2022, February 17). Food and Drug Administration. https://web.archive.org/web/20230102065900/https://www.fda.gov/about-fda/fda-commissioner
Center for Drug Evaluation and Research. (2022, August 27). Prescription Drug User Fee Amendments. Food and Drug Administration. https://web.archive.org/web/20230120033255/https://www.fda.gov/industry/fda-user-fee-programs/prescription-drug-user-fee-amendments
LaMattina, J. (2018, June 28). The biopharmaceutical industry provides 75% of the FDA’s drug review budget. Is this a problem? Forbes. http://archive.today/2023.01.22-210302/https://www.forbes.com/sites/johnlamattina/2018/06/28/the-biopharmaceutical-industry-provides-75-of-t he-fdas-drug-review-budget-is-this-a-problem/?sh=6c0ae09549ec
Woodcock, J., & Junod, S. (2012, June 8). PDUFA lays the foundation: launching into the era of user fee acts. Food and Drug Administration. https://web.archive.org/web/20170722143500/https://www.fda.gov/AboutFDA/WhatWeDo/History/Overviews/ucm305697.htm
Chen, C. (2018, June 26). FDA repays industry by rushing risky drugs to market. ProPublica. http://archive.today/2021.08.27-203148/https://www.propublica.org/article/fda-repays-industry-by-rushing-risky-drugs-to-market
Fact sheet: FDA at a glance. (2019, October 18). Food and Drug Administration. https://web.archive.org/web/20191203185354/https://www.fda.gov/about-fda/fda-basics/fact-sheet-fda-glance
FDA at a Glance (p. 2). (2020). Food and Drug Administration. https://web.archive.org/web/20221005204150/https://www.fda.gov/media/143704/download
Fact sheet: FDA at a glance. (2022, August 17). Food and Drug Administration. https://web.archive.org/web/20221005183101/https://www.fda.gov/about-fda/fda-basics/fact-sheet-fda-glance
Harkins, E. (2021, May 6). Re: BLA 125742 - COVID-19 mRNA Vaccine (BNT162/PF-07302048) - Part 1 of the Original Submission – Rolling Biologics License Application (BLA) - Request for Priority Review Designation. Public Health and Medical Professionals for Transparency; Pfizer Global Regulatory Affairs. https://web.archive.org/web/20230122214616/https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M1_cover.pdf
Gruber, M. F. (2020). Emergency Use Authorization (EUA) for an unapproved product review memorandum: Pfizer-BioNTech COVID-19 Vaccine/ BNT162b2. Food and Drug Administration. https://web.archive.org/web/20230803085800/https://www.fda.gov/media/144416/download
Beachy, S. H., Alper, J., Hackmann, M., & Addie, S. (2020, April 9). Integrating gene-based therapies into clinical practice: exploring long-term clinical follow-up of patients. National Center for Biotechnology Information; National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK559946/
110th Congress. (2007, September 27). Food and Drug Administration Amendments Act of 2007. U.S. Government Printing Office. https://web.archive.org/web/20221005184742/https://www.govinfo.gov/content/pkg/PLAW-110publ85/html/PLAW-110publ85.htm
Programs. Reagan-Udall Foundation. Retrieved January 13, 2023, from https://web.archive.org/web/20230113233812/https://reaganudall.org/programs
Sigal, E. V., & Hahn, S. M. (2020, February 19). Annual Report 2019. Reagan-Udall Foundation for the FDA. https://web.archive.org/web/20220712071448/https://reaganudall.org/sites/default/files/2022-02/Reagan-Udall_Annual%20Report%2020219_final_corrected.pdf
Winckler, S. C., & Woodcock, J. (2021, July 1). Annual Report 2020. Reagan-Udall Foundation for the FDA. https://web.archive.org/web/20220712065838/https://reaganudall.org/sites/default/files/2021-07/7-1-21_Reagan-Udall_AR.pdf
Sigal, E. V., Winckler, S. C., & Califf, R. M. (2022, May). Annual Report 2021. Reagan-Udall Foundation for the FDA. https://web.archive.org/web/20230113233656/https://reaganudall.org/sites/default/files/2022-05/Reagan-Udall_Annual%20Report%202022_final_Web.pdf
Foley, K. E., & Cancryn, A. (2022, August 4). Clinton-era FDA commissioner to lead external review of key agency offices. POLITICO. http://archive.today/2023.06.27-210118/https://www.politico.com/news/2022/08/04/fda-jane-henney-reagan-udall-00049977
Moderna. Flagship Pioneering. Retrieved September 15, 2022, from http://archive.today/2022.09.15-150329/https://www.flagshippioneering.com/companies/moderna
BIO member directory. Biotechnology Innovation Organization. Retrieved September 30, 2022, from https://www.bio.org/bio-member-directory
Member companies. Consumer Healthcare Products Association. Retrieved October 5, 2022, from https://my.chpa.org/Directories/Member-Companies
About. PhRMA. Retrieved April 5, 2022, from https://www.phrma.org/about
Birn, A.-E., & Richter, J. (2017, May 16). U.S. philanthrocapitalism and the global health agenda: the Rockefeller and Gates Foundations, past and present. Global Policy Forum. https://archive.globalpolicy.org/component/content/article/270-general/52947-us-philanthrocapitalism-and-the-global-health-agenda-the-rockefeller-and-gates-foundations-past-and-present.html
Pfizer. Bill & Melinda Gates Foundation Strategic Investment Fund. Retrieved June 22, 2021, from http://archive.today/2021.06.22-183302/https://sif.gatesfoundation.org/investments/pfizer/
Bill & Melinda Gates Foundation — Advancing an mRNA-based antibody combination to help prevent HIV infection. Moderna. Retrieved March 21, 2022, from http://archive.today/2021.08.23-023928/https://www.modernatx.com/ecosystem/strategic-collaborators/foundations-advancing-mrna-science-and-research
Bill and Melinda Gates Foundation donations made to Janssen Vaccines & Prevention B.V. Vipul Naik. Retrieved March 29, 2023, from http://archive.today/2023.03.29-052407/https://donations.vipulnaik.com/donorDonee.php?donor=Bill+and+Melinda+Gates+Foundation&donee=Janssen+Vaccines+%26+Prevention+B.V.
Canellis, D. (2020, June 5). Bill Gates commits $750M to help Oxford vaccinate the world against COVID-19. TNW | Fintech-Ecommerce. http://archive.today/2023.02.25-123239/https://thenextweb.com/news/bill-gates-covid-coronavirus-vaccine-750-million-oxford-azd1222
Boehler, M. (2019, September 4). BioNTech announces new collaboration to develop HIV and tuberculosis programs. BioNTech. http://archive.today/2022.01.03-191438/https://investors.biontech.de/news-releases/news-release-details/biontech-announces-new-collaboration-develop-hiv-and
About our Alliance. Gavi, the Vaccine Alliance. Retrieved May 16, 2021, from http://archive.today/2021.05.16-121329/https://www.gavi.org/our-alliance/about
CEPI investment overview. (2022, July 6). Coalition for Epidemic Preparedness Innovations. https://web.archive.org/web/20220829190949/https://cepi.net/wp-content/uploads/2022/02/2022_07_06-CEPI-Investment-Overview.pdf
Bill & Melinda Gates Foundation. The Global Fund. Retrieved November 25, 2022, from http://archive.today/2022.11.25-210616/https://www.theglobalfund.org/en/private-ngo-partners/resource-mobilization/bill-melinda-gates-foundation/
Banco, E., Furlong, A., & Pfahler, L. (2022, September 14). How Bill Gates and partners used their clout to control the global Covid response — with little oversight. POLITICO. http://archive.today/2023.08.05-080416/https://www.politico.com/news/2022/09/14/global-covid-pandemic-response-bill-gates-partners-00053969
Belvedere, M. J. (2019, January 23). Bill Gates: My “best investment” turned $10 billion into $200 billion worth of economic benefit. CNBC. https://web.archive.org/web/20230803043118/https://www.cnbc.com/2019/01/23/bill-gates-turns-10-billion-into-200-billion-worth-of-economic-benefit.html
Working Group on Readying Populations for COVID-19 Vaccine. Johns Hopkins Center for Health Security. Retrieved October 5, 2022, from http://archive.today/2021.04.04-023329/https://www.centerforhealthsecurity.org/our-work/Center-projects/CONVERGE.html
Our history. Johns Hopkins Center for Health Security. Retrieved May 10, 2023, from http://archive.today/2023.05.10-002117/https://centerforhealthsecurity.org/who-we-are/history-of-the-center-for-health-security
Event 201. Johns Hopkins Center for Health Security. Retrieved July 9, 2023, from http://archive.today/2023.07.09-062022/https://centerforhealthsecurity.org/our-work/tabletop-exercises/event-201-pandemic-tabletop-exercise
Schoch-Spana, M., Brunson, E. K., Shearer, M. P., Ravi, S., Sell, T. K., Chandler, H., & Gronvall, G. K. (2017, October). The SPARS pandemic, 2025-2028: A futuristic scenario for public health risk communicators. Johns Hopkins Center for Health Security. https://web.archive.org/web/20180330033845/https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2017/spars-pandemic-scenario.pdf
Working Group on Readying Populations for COVID-19 Vaccine. Johns Hopkins Center for Health Security. Retrieved May 10, 2023, from http://archive.today/2023.05.10-003623/https://centerforhealthsecurity.org/our-work/research-projects/working-group-on-readying-populations-for-covid-19-vaccine
Yannuzzi, R. E. In-Q-Tel: A new partnership between the CIA and the private sector. Central Intelligence Agency. Retrieved August 16, 2000, from https://web.archive.org/web/20000816205529/http://www.cia.gov/cia/publications/inqtel/
Brunson, E. K., & Schoch-Spana, M. (2020). A social and behavioral research agenda to facilitate COVID-19 vaccine uptake in the United States. Health Security, 18(4). https://doi.org/10.1089/hs.2020.0106
Bhat, A., Browning-McNee, L. A., Ghauri, K., & Winckler, S. (2021). COVID-19 vaccine confidence project. Journal of the American Pharmacists Association. https://doi.org/10.1016/j.japh.2021.06.006
Chang, A., & Miller, M. (2021, May 25). Johns Hopkins Center for Health Security receives a grant from the Rockefeller Foundation to deliver Covid-19 Response Research as part of the Exemplars in Global Health program. The Rockefeller Foundation. https://web.archive.org/web/20230605065933/https://www.rockefellerfoundation.org/news/johns-hopkins-center-for-health-security-receives-a-grant-from-the-rockefeller-foundation-to-deliver-covid-19-response-research-as-part-of-the-exemplars-in-global-health-program/
O’Shea, D., & Pettengill, L. (1999, May). Gates Foundations give Johns Hopkins $20 million gift to School of Public Health for Population, Reproductive Health Institute. Bill & Melinda Gates Foundation. http://archive.today/2021.08.26-074051/https://www.gatesfoundation.org/ideas/media-center/press-releases/1999/05/johns-hopkins-university-school-of-public-health
Alexopulos, N. (2018, January 22). Center for Health Security receives $399K grant from Rockefeller Foundation to create scalable checklist for nations to assess health system resilience and a guide to making improvements. Johns Hopkins Center for Health Security. https://web.archive.org/web/20230711213712/https://centerforhealthsecurity.org/2018/center-for-health-security-receives-399k-grant-from-rockefeller-foundation-0
BioNTech SE, & Pfizer. (2023, February 28). Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals. ClinicalTrials.gov. https://web.archive.org/web/20230420203001/https://clinicaltrials.gov/ct2/show/NCT04368728
COVID-19 team. Johns Hopkins Center for Immunization Research. Retrieved July 11, 2023, from https://web.archive.org/web/20230711215122/https://centerforimmunizationresearch.org/our-research/covid-19-team/
Adalja, A. A., Watson, M., Cicero, A., & Inglesby, T. (2019, April). Vaccine platforms: State of the field and looming challenges. Johns Hopkins Center for Health Security. https://web.archive.org/web/20230711204444/https://centerforhealthsecurity.org/sites/default/files/2022-12/190423-opp-platform-report.pdf
Home. COVID-19 Evidence Accelerator. Retrieved September 7, 2023, from https://web.archive.org/web/20230907235240/https://evidenceaccelerator.org/
Stewart, M., Rodriguez-Watson, C., Albayrak, A., Asubonteng, J., Belli, A., Brown, T., Cho, K., Das, R., Eldridge, E., Gatto, N., Gelman, A., Gerlovin, H., Goldberg, S. L., Hansen, E., Hirsch, J., Ho, Y.-L., Ip, A., Izano, M., Jones, J., & Justice, A. C. (2021). COVID-19 Evidence Accelerator: A parallel analysis to describe the use of Hydroxychloroquine with or without Azithromycin among hospitalized COVID-19 patients. PLOS ONE, 16(3), e0248128. https://doi.org/10.1371/journal.pone.0248128
@CovidAnalysis. HCQ for COVID-19: real-time analysis of all 551 studies. C19hcq: COVID-19 HCQ Treatment Analysis. Retrieved September 7, 2023, from https://c19hcq.org/
Tyson, B., Fareed, G., & Crawford, M. (2022). Overcoming the COVID-19 darkness: How two doctors successfully treated 7000 patients. Brian Tyson, M.D. and George C. Fareed, M.D. https://www.amazon.ca/Overcoming-COVID-19-Darkness-Successfully-Patients/dp/B09PVNF24K/
Leake, J., & McCullough, P. A. (2022). The courage to face COVID-19: Preventing hospitalization and death while battling the bio-pharmaceutical complex. Skyhorse Publishing. https://www.amazon.ca/COURAGE-FACE-COVID-19-Hospitalization-Bio-Pharmaceutical/dp/B09ZLVWMD9
Ison, M. G., Wolfe, C., & Boucher, H. W. (2020). Emergency Use Authorization of remdesivir. JAMA. https://doi.org/10.1001/jama.2020.8863
Tantibanchachai, C. (2021, December 23). Coronavirus (COVID-19) update: FDA authorizes additional oral antiviral for treatment of COVID-19 in certain adults. Food and Drug Administration. http://archive.today/2021.12.23-144625/https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain
Taylor, M., & Rowland, T. (2020, September 17). Lilly and Amgen announce manufacturing collaboration for COVID-19 antibody therapies. Amgen. http://archive.today/2022.01.12-213722/https://www.amgen.com/newsroom/press-releases/2020/09/lilly-and-amgen-announce-manufacturing-collaboration-for-covid-19-antibody-therapies
Bowie, A. (2020, August 19). Regenereon and Roche collaborate to significantly increase global supply of REGN-CoV2 investigational antibody cocktail for COVID-19. Regeneron Pharmaceuticals. http://archive.today/2023.05.11-184101/https://newsroom.regeneron.com/news-releases/news-release-details/regeneron-and-roche-collaborate-significantly-increase-global
Hinton, D. M. (2020, May 1). Remdesivir EUA Letter of Authorization. Food and Drug Administration. https://web.archive.org/web/20200501204823/https://www.fda.gov/media/137564/download
Alliance for a Stronger FDA lobbying profile. (2022, July 22). OpenSecrets. https://www.opensecrets.org/federal-lobbying/clients/summary?cycle=2021&id=D000046669
AstraZeneca calls for FDA funding increase. (2010, September 20). Pharmaceutical Technology. https://web.archive.org/web/20220817001606/https://www.pharmaceutical-technology.com/uncategorized/news96475-html/
List of members. (2022, March 4). Alliance for a Stronger FDA. http://archive.today/2022.07.08-045902/https://www.strengthenfda.org/members
Current members. Personalized Medicine Coalition. Retrieved April 6, 2022, from https://web.archive.org/web/20210815032705/https://personalizedmedicinecoalition.org/Members/Current_Members
Ibid (PhRMA).





