Government of Canada Misled Canadians on COVID-19 Adverse Event Reporting
Health Canada restricted ability to report vaccine injuries during pandemic
A new document produced in response to an Order Paper Question in Canadian Parliament has revealed a series of strange and upsetting actions taken by Health Canada to make it very, very hard for individuals to report suspected adverse events after receiving a COVID-19 vaccine product.
Introduction
During the declared COVID-19 pandemic, the Government of Canada expedited the development and approval of novel genetic vaccine products in order to rapidly deliver them to the Canadian population. This was the sole plan put in place from the get-go, with public health measures like lockdowns, “social distancing” and face masks put in place in order to buy time until needles could enter arms.

Prime Minister Justin Trudeau said as much right as Canada first entered “lockdown” mode:1
Our healthcare systems across the country are coping — for the time being. But we’re at a fork in the road between the best and the worst possible outcomes.
The best possible outcome is no easy path for any of us. The initial peak, the top of the curve, may be in late spring with the end of the first wave in the summer. As Dr. [Teresa] Tam explained, there will likely be smaller outbreaks for a number of months after that.
This will be the new normal… until a vaccine is developed.
But as we all know, this was an undertaking unlike any prior vaccine campaign. This was a process that went significantly quicker than usual, using technology and methodology never before attempted on a national population. As said so eloquently several months ago by Member of Parliament Anthony Housefather:2
Companies were being told to rush vaccine production and do testing in an unprecedented way, in a way that they don't normally do it.
These companies were exposed to a way higher liability in putting their products on the market than they normally would be, because they didn't do the type of testing that normally means these drugs take years to come to market. They did it all in less than year.
Housefather’s concern for the pharmaceutical companies in question is much appreciated by some, I’m sure. Indeed, the COVID-19 vaccine candidates were rushed through testing and production, which would be a risky enough proposition even if using traditional vaccine technologies.
But that’s not what these were. All four COVID-19 vaccine products offered in Canada were built on a novel gene therapy platform, be they based on mRNA (Pfizer-BioNTech and Moderna) or adenoviral vector (Janssen and AstraZeneca).
Both the speed and the novelty of these products necessitates a heightened degree of scrutiny over the possible harms caused to those who receive them. However, a new report provided by the Government of Canada in response to an Order Paper Question in Parliament suggests that multiple agencies within the Government of Canada made decisions that severely compromised the nation’s ability to capture and analyze adverse event reports.
Last week, Tamara Ugolini of Rebel News highlighted Health Canada’s admission that they are “aware of the ongoing release” of “documents by the US Food and Drug Administration” — specifically, the infamous report titled “5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162B2) Received Through 28-FEB-2021,” which included nine pages of “adverse events of special interest”3 — the overwhelming majority of which were not disclosed as possible risks to those considering taking the shot.
Health Canada and its sister agency, the Public Health Agency of Canada, insist that the report “did not identify new safety concerns and was consistent with the known safety profile” of the Pfizer-BioNTech COVID-19 vaccine, and therefore, “no changes to the terms of authorization or the labelling of the vaccine were required.”4
But what has not yet been reported is the response to a second Order Paper Question (OPQ), which details a specific set of actions taken by the Government of Canada to restrict the adverse event reporting process in Canada, resulting in confusion among health care practitioners and the general public alike, and severely limiting the ability for regulators to identify safety signals as people began getting hurt and dying in sudden and unexpected ways.
I will share this with you now, and explain why I believe it shows that the government is misleading Canadians about the true nature of adverse event data in Canada.
You ready? Let’s go.
The Order Paper Question
For those who want to start with seeing the Order Paper Question itself, here you go. Take a look over, absorb, and then return to this report. Keep the OPQ open in a separate tab if you like, as we will follow along with it more closely in a few paragraphs.
Here was the specific question asked by Conservative Member of Parliament Colin Carrie on May 1, 2023:
Before we can begin our analysis of the government’s answers, we first need to establish some definitions and a baseline understanding of how adverse event reporting is set up in Canada, particularly compared to other countries.
What is pharmacovigilance?

As defined by the World Health Organization, “pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine/vaccine related problem.”5
Adverse event reporting systems around the world
Many such systems exist around the world. Some of them are focused specifically on vaccines, while others keep their eye on medical products more broadly. A few examples that people may be aware of are:
Vaccine Adverse Event Reporting System (VAERS) in the United States, run jointly by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA);6
Yellow Card scheme in the United Kingdom, run by the Medicines & Healthcare Products Regulatory Agency (MHRA);
EudraVigilance in the European Union; and
VigiAccess, run by the World Health Organization.
To be clear, no adverse event reporting system is perfect, nor are they intended to be. Each of these systems has their share of problems, but for all intents and purposes, they are functioning adverse event reporting systems that have successfully identified safety signals, resulting in the drug or vaccine in question being restricted or pulled from the market altogether.
A fundamental principle of pharmacovigilance is that one does not need to be certain that a given drug or vaccine caused the harm, just that there is a correlation. This has been a huge point of contention during the COVID-19 era, with ‘fact-checks’ aplenty asserting that correlation doesn’t equal causation, particularly in response to concerns raised around VAERS.7

The result of this is that the general public and regulators alike all seem to believe that VAERS, therefore, can’t possibly offer any meaningful information. This is a dangerous attitude, as it risks eliminating the possibility of detecting a safety signal when it inevitably arises.
As my friend
testified to in European Parliament several weeks ago, “VAERS works.” Of course, “it works when it’s being looked at,” which the FDA and CDC have apparently decided not to do.8 But thanks to Jessica’s hard work, many others are looking at VAERS, and allowing it to fulfil its purpose nonetheless.
For their part, the Medicines & Healthcare Products Regulatory Agency even rolled out a dedicated Coronavirus Yellow Card reporting site specifically intended to capture adverse events to COVID-19 vaccines and therapeutics in the United Kingdom.
So, what about Canada?
Pharmacovigilance in Canada
Canada’s federal health bureaucracy is split across several agencies and sub-agencies sitting within the so-called “Health portfolio,” all of which report to our Minister of Health.9 The two primary agencies for health regulation and guidance are Health Canada and the Public Health Agency of Canada (PHAC).

Health Canada is responsible for national health policy, including “regulating drugs and health products to support public safety.”10 It is the agency responsible for authorizing the COVID-19 vaccines starting in December 2020, doing so under an interim order issued in September 2020 titled “Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19.”11 You can think of Health Canada as our equivalent to the Food and Drug Administration (FDA), and the interim order as comparable in some ways to an Emergency Use Authorization (EUA).

The Public Health Agency of Canada (PHAC) is responsible for public health, emergency preparedness and response, and infectious and chronic disease control and prevention. It does this in large part by preparing and disseminating guidelines, as opposed to through regulation. PHAC is comparable to the Centers for Disease Control and Prevention (CDC) in the United States.
Once Health Canada authorized the COVID-19 vaccines under interim order, they were turned over to PHAC to generate official recommendations for their use.

This task was delegated to the National Advisory Committee on Immunization (NACI),12 a so-called “arm’s length” body that is chock-full of conflicts of interest — a topic I fleshed out in a reference book last year called National Advisory Committee on Immunization: COVID-19 Report. You can grab a copy on Amazon if you’d like to have it on hand.
Health Canada and PHAC both reviewed the safety information provided by Pfizer in their application, which was limited to the clinical trial data captured in the Phase I/II studies, and preliminary Phase III data (which, as noted in the Summary Basis of Decision, was still ongoing at the time of authorization.)13
But once the shots were deployed into the population, “post-market surveillance” becomes the new focus. In other words, the capture of adverse event reports from patients or their health care practitioners, as opposed to relying solely on data provided by the pharmaceutical companies themselves.
This is where we will begin to follow along with the Order Paper Question that underlies this report.14
Pharmacovigilance related to Adverse Events Following Immunization (AEFI) is a shared responsibility between Health Canada and PHAC. But the way in which these two agencies receive and process reports is not uniform, nor are their legal reporting obligations. And in many cases, it’s not entirely clear to what degree their adverse event reports are compared or combined. Let’s take a look at the specific programs each agency runs to capture adverse events.
Canada Vigilance Program

Health Canada runs the Canada Vigilance Program (CVP), which the agency describes as its “post-market surveillance program that collects and assesses reports of suspected adverse reactions to health products marketed in Canada.”15 The scope of the program spans drugs, medical devices, vaccines, natural health products, cannabis and veterinary drugs.16
The data collected through the CVP is published in the Canada Vigilance Adverse Reaction Online Database, searchable in a fashion similar to VAERS’ “Wonder System” in the United States.
Also like VAERS, the Canada Vigilance Program receives adverse event reports from a wide range of types of reporters — including the general public. “Market authorization holders” (e.g. Pfizer, Moderna, Janssen, AstraZeneca) are required by law to report adverse events to CVP, which are identified through the companies’ own post-marketing surveillance. Health care professionals and hospitals can submit reports to CVP, but as we’ll see shortly, it’s not clear how often they do.
As with the adverse event reporting systems described above, CVP is not intended to be perfect. Visitors to the database are warned that the CVP:17
…is a spontaneous reporting system that is designed to detect signals of potential health product safety issues during the post-market period. The data is collected primarily by a spontaneous surveillance system in which adverse reactions to health products are reported on a voluntary basis. However, Health Canada is aware that adverse reactions are often under-reported to both voluntary and mandatory spontaneous surveillance systems.
This is another fundamental principle of pharmacovigilance systems, as formalized in the now well-known study by Harvard Pilgrim Health Care which found that “adverse events from drugs and vaccines are common, but underreported,” and more specifically, “fewer than 1% of vaccine adverse events are reported” in the United States.18
Furthermore, visitors to the CVP website are warned, the database “contains only a small proportion of adverse reactions reported following receipt of vaccines, and is reflective of serious reactions reported to market authorization holders as required under the Food and Drugs Act. The majority of reports of these reaction (sic) are submitted to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS).”
Canadian Adverse Events Following Immunization Surveillance System
Enter adverse event reporting system #2.

The Public Health Agency of Canada runs the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS). Unlike the Canada Vigilance Program, which captures adverse events from a range of health-related products, CAEFISS is specifically focused on vaccine reactions.
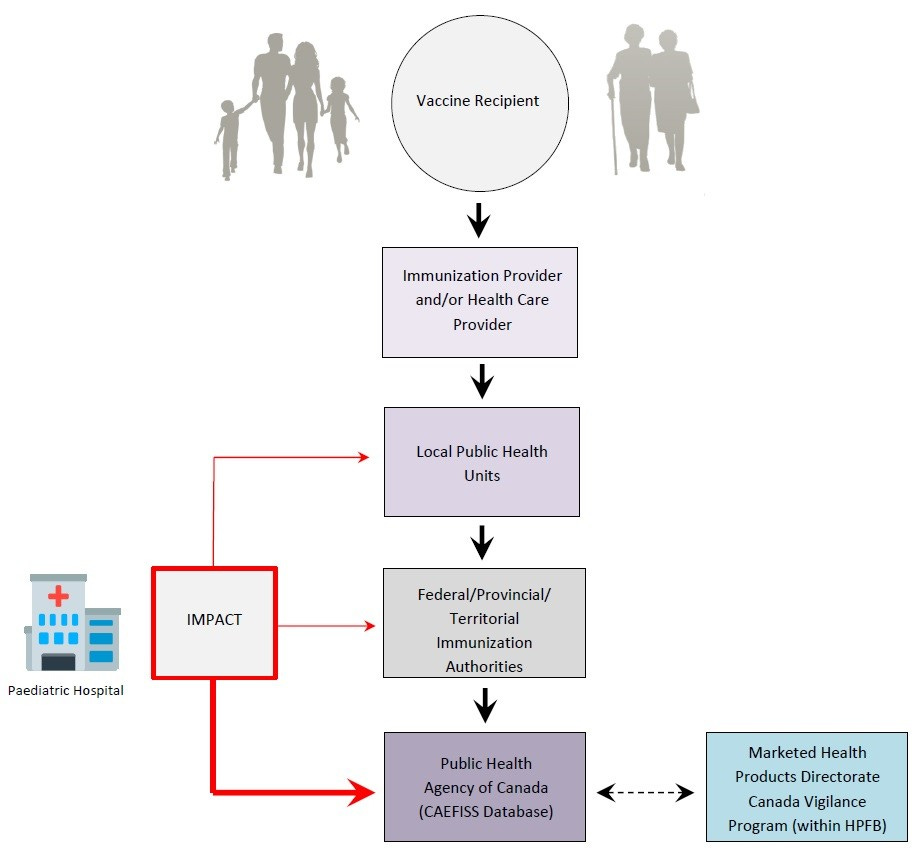
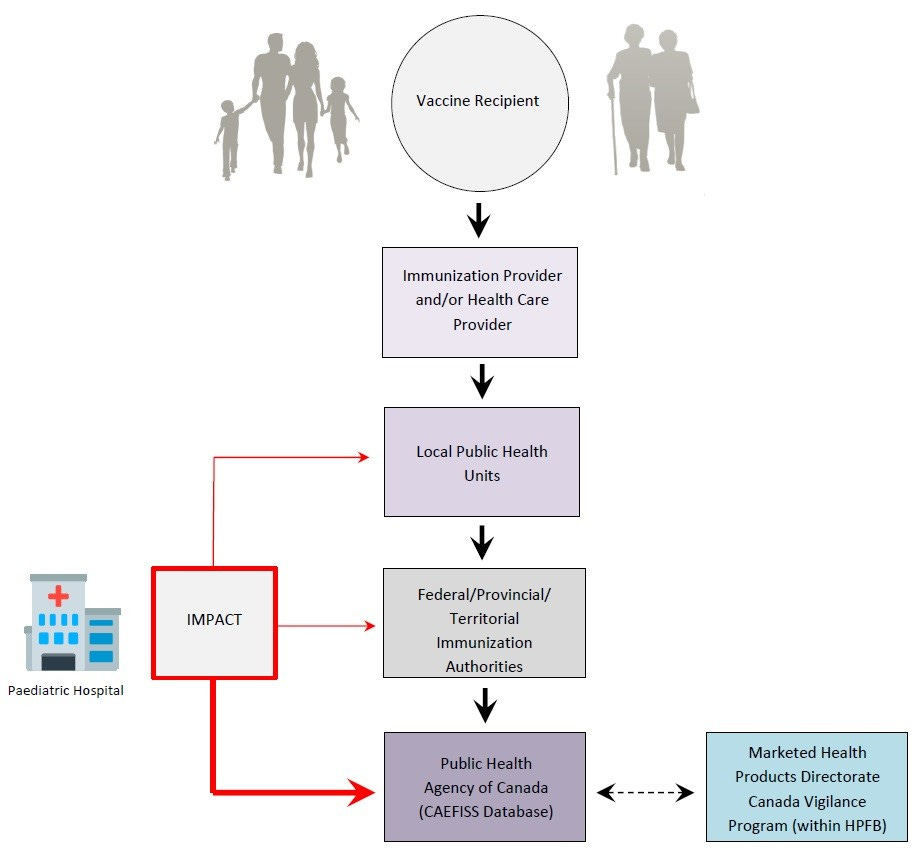
Also unlike CVP, CAEFISS does not accept adverse event reports directly from the general public. Instead, reports are generated by the “immunization provider,” or healthcare provider, who submit them to a Local Public Health Unit. If accepted as legitimate by the unit, the report is then passed up to a “Federal/Provincial/Territorial Immunization Authority” for further review. Only with the blessing of this level of agency does the adverse event report ever reach the Public Health Agency of Canada for processing into the CAEFISS database.19
Furthermore, CAEFISS engages in both passive and active surveillance. “Passive surveillance” is when reports are submitted to the agency by concerned parties as events occur, whereas “active surveillance” involves proactively seeking out data to investigate a possible safety signal.
Changes to reporting protocols during COVID-19
As I described in a previous article, there is a specific webpage where individuals are likely to wind up if they’re attempting to report an adverse event to a vaccine, aptly titled “Report a side effect to a vaccine: Consumers.” This is where consumers have historically been directed if they want to report an adverse event to Health Canada.
In their response to Colin Carrie’s OPQ, Health Canada describes a decision made in December 2019 to update the page to “enhance its user-friendliness and reflect the mandatory reporting of serious adverse events of drugs and medical devices by hospitals.” This version of the page featured a nice-looking, easy-to-use button right at the top labelled “Report a side effect,” as well as an option to mail the report to a regional office.

Helpfully, the page reminds people that they can ask their health care provider for help, but does not require people to do so if they’re comfortable submitting the report on their own. Options are good!
This is how the page remained for the entirety of the following year. But on December 1, 2020, the webpage was updated once again. The “report a side effect” button was removed entirely, and the information on the page was fundamentally changed.

Now, instead of being able to report their side effect to Health Canada’s Canada Vigilance Program, visitors to the site were told that they needed to have their health care provider fill out a form and “submit it on your behalf to your local public health unit.”
This message is hammered home with a graphic that appeared for the first time on the page with this new update, which clearly indicates the ‘vaccine recipient’ is required to go through their health care provider. The Canada Vigilance Program is now presented as being for ‘manufacturers’ and ‘Market Authorization Holders.’
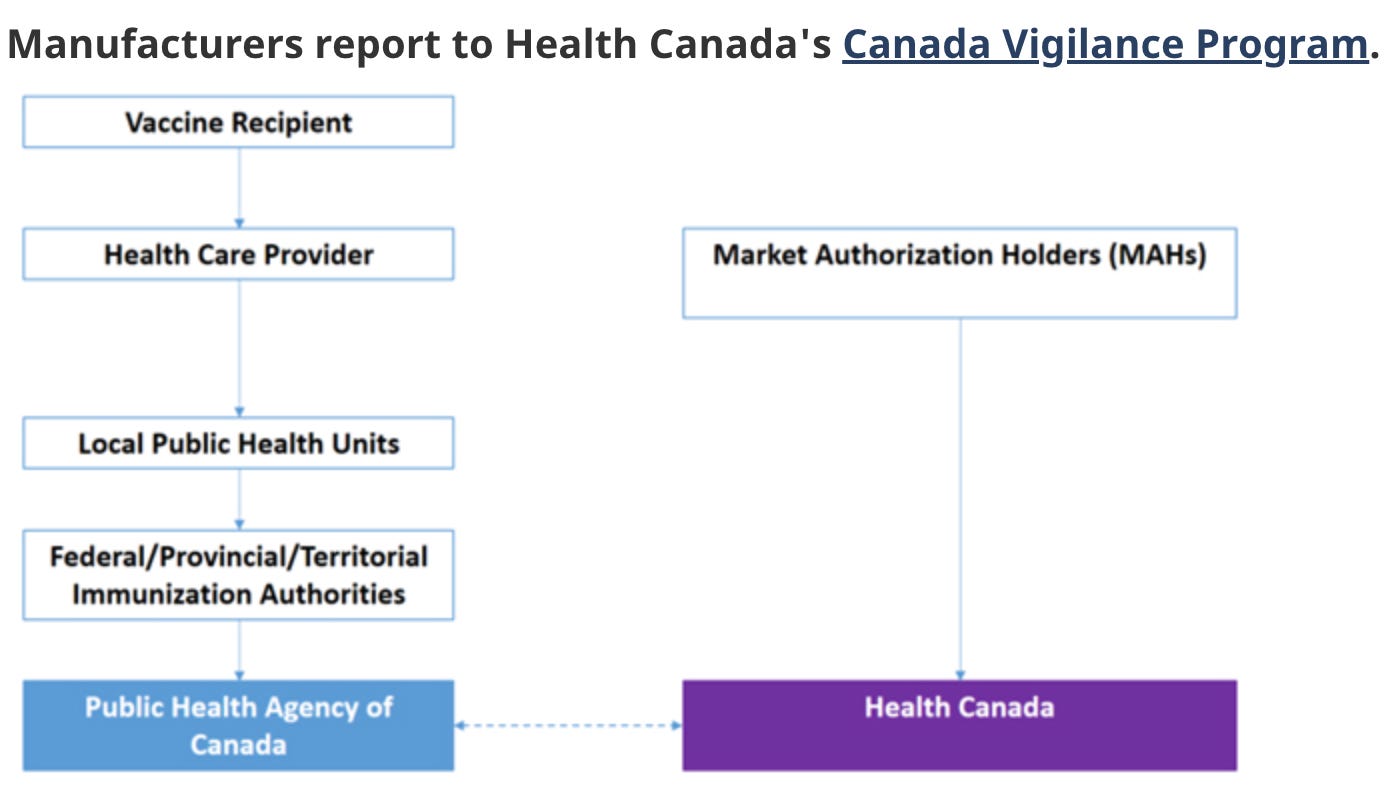
In other words, this page, run by Health Canada and dedicated to the Canada Vigilance Program, was now redirecting all potential adverse event reports to CAEFISS — an entirely different system, run by an entirely different agency. The graphics are nearly identical in function, but different in design, suggesting time and effort were spent to create the new, less-informative graphic.
Why would they do this?
According to Health Canada, at some point after the December 2019 update, an unidentified “provincial stakeholder” advised the agency that rather than accepting adverse event reports through the Canada Vigilance Program, “reporting to CAEFISS was the correct path for vaccines in [provinces and territories] and should be used exclusively for [provincial/territorial] health authorities during the pandemic.”
There are serious problems with this explanation. First of all, the concerns raised by the “provincial stakeholder” appear to have been strictly related to adverse event reporting by entities operating in the provincial/territorial health system, which, in addition to health authorities, would presumably also include hospital networks.
But the web portal they altered was the one specifically intended for use by consumers. If hospital networks and health authorities were reporting to both CVP and CAEFISS, and the stakeholder wanted them to only report to CAEFISS, then would the appropriate solution not be to take it up with the hospital networks and health authorities? Was this “consumers” portal the same one being used by medical professionals, contrary to what the page itself suggests?
Furthermore, if the idea was to streamline the adverse event reporting process “during the pandemic,” then why was this change made 8 months after the pandemic was declared? Even if people weren’t getting vaccinated as much from March to November 2020, vaccines for various diseases were still being given in Canada, and any adverse event reports would have continued to be processed through the Canada Vigilance Program as they came in.
It was only when the COVID-19 vaccines were within weeks of nationwide distribution that this “simplifying the AEFI reporting process” occurred.
By making this change, individuals who previously would have submitted their adverse events directly to Health Canada were now forced to go through the highly indirect, much more laborious, multi-level review process run by PHAC. At a bare minimum, this would guarantee that adverse event data would be collected at a significantly slower rate, reducing the likelihood of identifying a safety signal from the novel COVID-19 genetic vaccines early. This means that millions of Canadians were unnecessarily and recklessly put at greater potential risk of injury or death from a given adverse event, which the Canada Vigilance Program would be far quicker to identify.
What were doctors told to do?
But it’s worse than that, because for an adverse event to ever reach the CAEFISS database, multiple levels of people needed to agree to send it further up the chain. At the very first level, physicians and health care providers across the country were not informed about the full range of adverse events that their patients may suffer, with only a slim range of injuries recognized as legitimate grounds to even consider filing a report.
Dr. Patrick Phillips, an emergency physician in Ontario, filed several cases of suspected AEFIs to his local public health unit, which included patients suffering facial numbness, loss of arm functionality, abdominal pain and vomiting, heart palpitations, large rashes, severe fatigue, severe vertigo and tinnitus, just to name a few20 — all of which were known to Health Canada as adverse events of special interest related to the COVID-19 vaccine products. Well, assuming they did, indeed, review the post-marketing report from Pfizer, as they told Colin Carrie.21
All of Dr. Phillips’ reports were rejected by the Timiskaming Health Unit on the basis that they did “not meet the criteria for AEFI.”22 CEO of the unit, Glenn Corneil, told Phillips that he should review the Provincial Case Definitions for Diseases of Public Health Significance for AEFI.
The document is available for you to read here. As far as I can tell, there’s nothing in this document inconsistent with the adverse events Dr. Phillips observed and reported on behalf of his patients. But note that the document in question had not been updated since December 2020, before any adverse event data had been generated from the Canadian COVID-19 vaccine rollout. The Pfizer post-marketing surveillance report was dated February 2021, and Dr. Phillips submitted his adverse event reports in March/April 2021.
Timiskaming Public Health Unit put their hands over their ears and said, “thanks, but we don’t want to know.” Then they took back the “thanks.”

Dr. Phillips’ license was revoked three weeks ago, after more than a year of legal defence.23

Meanwhile, in British Columbia, Dr. Charles Hoffe observed his own patients suffering sudden, severe and unexplained health consequences following their receipt of a Moderna genetic vaccine. In his April 5, 2021 open letter to BC’s Provincial Health Officer, Dr. Bonnie Henry, Hoffe pointed out that the adverse event reporting form available from the province “was clearly designed for conventional vaccines,” as it did “not even have any place to report vaccine injuries of the nature and severity that we are seeing from this new mRNA therapy.”24 This was particularly alarming because these adverse events were “going almost entirely unreported, by those responsible for the vaccine rollout,” and further pointing out that the under-reporting of adverse events was a problem that existed even prior to COVID-19.

An open information request to the Government of British Columbia later revealed that rather than follow up on the reported adverse events among Dr. Hoffe’s patients, the Ministry of Health went into public relations damage control mode. Carol Fenton, the Medical Health Officer for the province’s Interior Health Authority, assured Henry and colleagues that she was “in the process of crafting a response with our communications department to try and mitigate the harm.”25
What did the communications department come up with?
Read the full article in Kamloops This Week.
Health Canada’s misdirection
To add insult to injury, Health Canada asserts that “reporting directly to CVP was still possible through phone, fax, email” — and, presumably, smoke signal, carrier pigeon, interpretive dance, singing gram and pantomime.
But that would require the patient to be aware of the Canada Vigilance Program, well enough so that they wouldn’t need to do any research in order to identify the now harder-to-find phone number or email address. It would also require that the patient not talk to their doctor first, as the vast majority of doctors would not be able to successfully submit their adverse event report even if they wanted to. This, despite there being a legal obligation to report all suspected adverse events, as was reiterated to Dr. Phillips in the letter reprimanding him for reporting “invalid” adverse events, and repeated throughout Health Canada’s OPQ response.
And if Health Canada is saying that reporting through CVP was still an option through which they were happy to receive adverse event reports to vaccines, then doesn’t that contradict the idea that this change was made in order to send all vaccine reports to CAEFISS instead? You know, because “reporting to CAEFISS was the correct path for vaccines”?
Health Canada continues, however, by admitting their removal of the “online vaccine reporting process may have caused confusion,” for exactly the reasons I articulated above. One might then presume that this led to a fairly quick fix of the webpage to clear up any confusion and ensure that the COVID-19 vaccine adverse event data was being collected as required, right?
Nope.
Needless to say, I was flabbergasted to read this. Nonetheless, the page was, indeed, edited once again on February 28, 2023 to reinstate the “Report a side effect” button, and other helpful information.

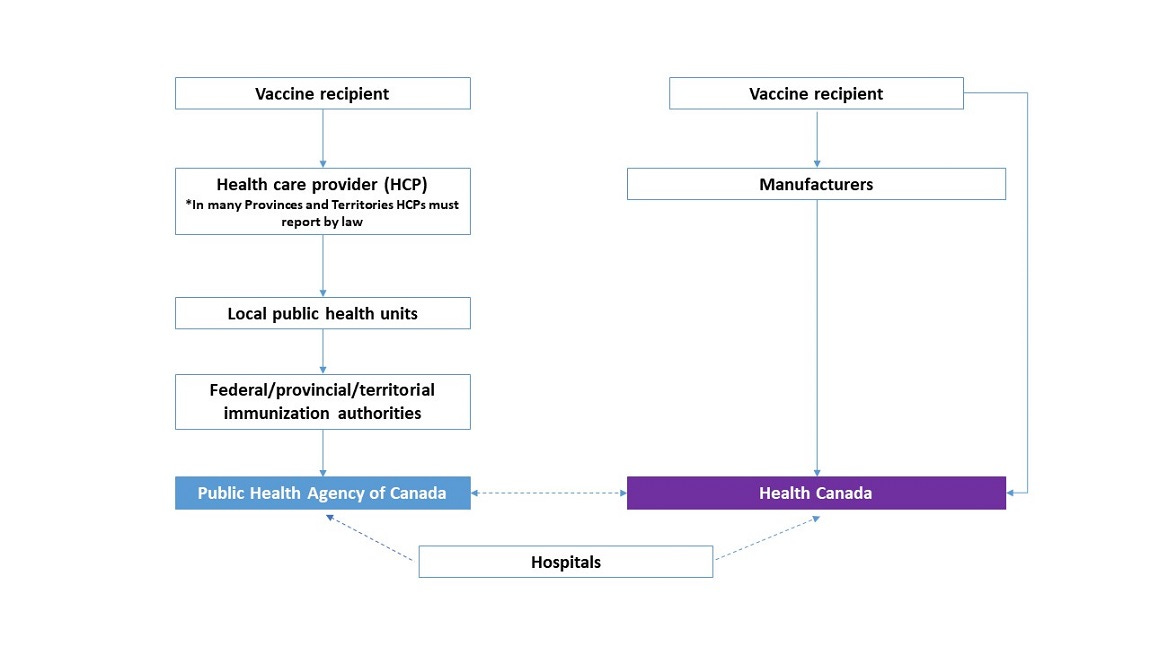
Once again, the webpage features a new graphic that completely contradicts everything that came before it. Now the vaccine recipient has not one, but three different outlets where they can report their adverse events: their health care provider, Health Canada, or the manufacturers themselves. Furthermore, Health Canada is now apparently very comfortable doubling down on the fact that health care providers “must report by law” to their local public health unit.
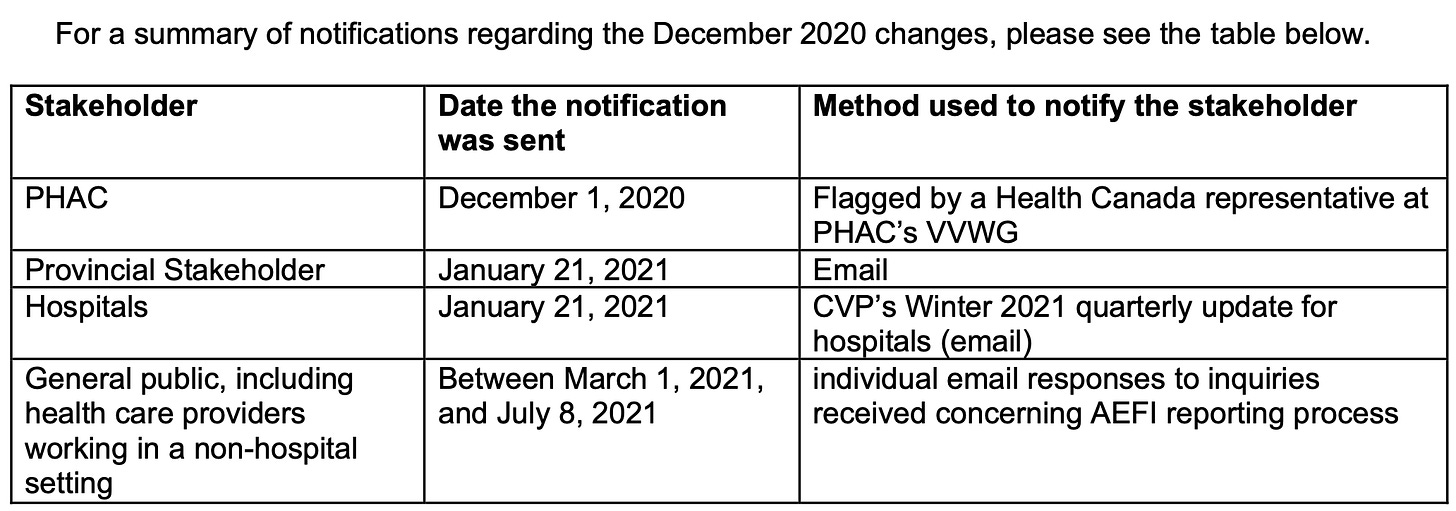
In a table provided in the OPQ response laying out when Health Canada engaged with “stakeholders” about the December 2020 changes, we see a date range of March 1 to July 8, 2021, during which “individual email responses to inquiries received concerning AEFI reporting process” were sent out.
Let’s lay out a quick timeline:
December 2019: Health Canada makes their online reporting form to CVP easier to use for consumers. Hooray!
Sometime between March-November 2020: An unnamed “provincial stakeholder” makes “comments” that all vaccine reports should go through CAEFISS during the pandemic.
December 2020: Health Canada removes the direct reporting option from their vaccine reporting webpage for CVP, changing it to redirect everyone to CAEFISS.
March 2021 - July 2021: Doctors and members of the general public complain/express their concerns to Health Canada about the AEFI process.
February 28, 2023: Health Canada “reinstated the direct link to Health Canada’s online reporting form.”
It took Health Canada two years to reinstate the direct reporting option following the first reported sign that it was causing problems. Two. Freaking. Years. And guess what? They didn’t tell anybody outside of the government that they did it.
I happen to know that Health Canada is not telling the truth here — at least not the whole truth. I want to be more explicit, but out of respect for the team I work with behind the scenes, I will wait until our pending Access to Information Request on this issue is completed and safely in our hands. If I understand correctly, this may establish some currently missing facts, such as which province’s “stakeholder” is allegedly responsible for this mess.
Health Canada makes clear that no money was spent educating the public about the newly-reinstated direct reporting ability, “given the voluntary nature of reports from the public.” In other words, consumers are not legally required to report adverse events, therefore no effort was made to educate the public on the fact that such a system even existed.
You know who else isn’t legally required to report adverse events to Health Canada? The Public Health Agency of Canada.
What does that even mean? Do they share data, or not? If so, to what degree? How are we supposed to understand the safety data presented for the COVID-19 vaccines in Canada?
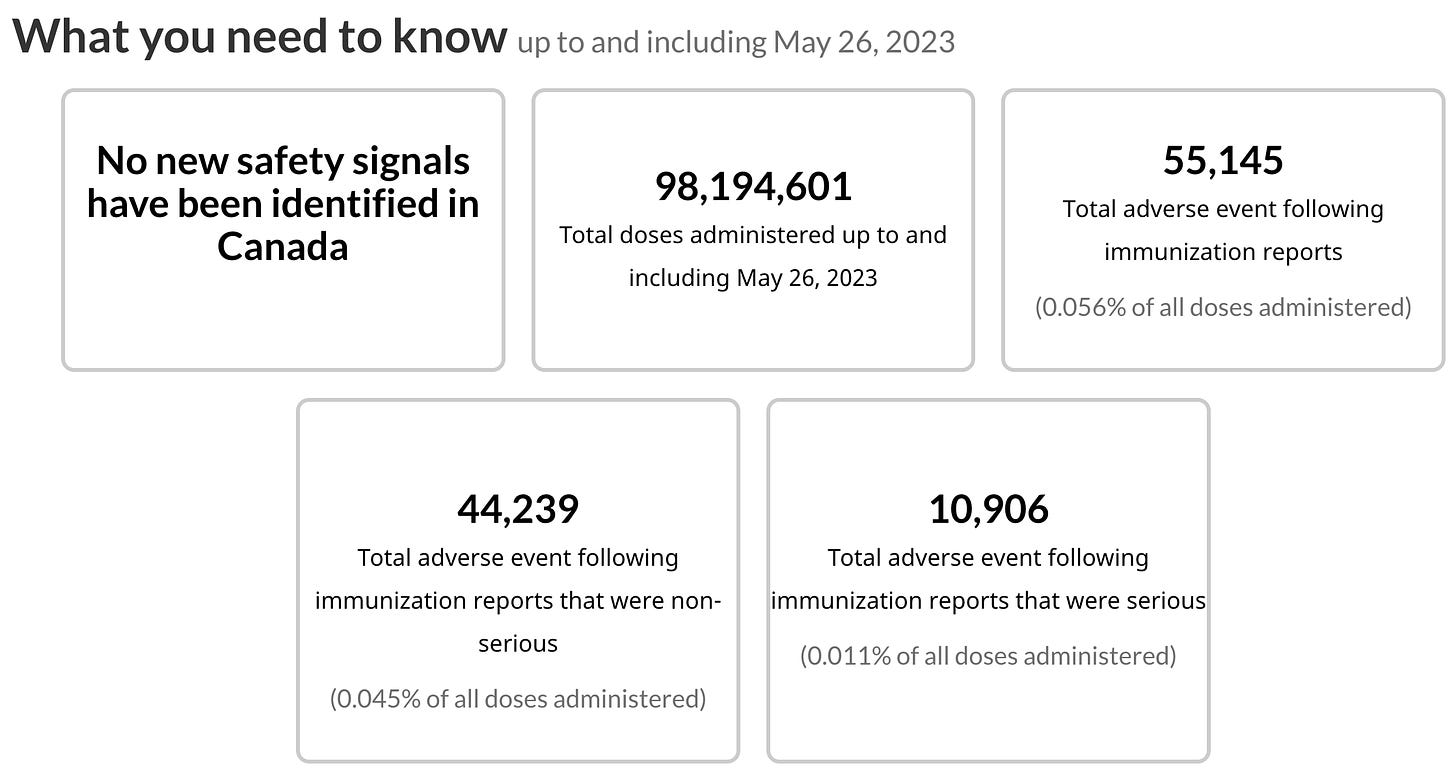
When you visit the webpage for Canada’s COVID-19 vaccine adverse event data, there is a note that explains that the data is generated with “combined numbers from both CAEFISS and the Canada Vigilance program,” which are “carefully merged together.” Still, they caution, “the individual programs are subject to different reporting requirements and definitions,” and for this reason and others, the data “may not accurately represent national COVID-19 vaccine adverse events following immunization.”26
Is it possible that because the Public Health Agency of Canada is not under any obligation to share the CAEFISS data with Health Canada, PHAC could choose to withhold reports based on a criteria of their choosing? The disclaimers on the data webpage certainly seem to allow for that option.
Perceived conflicts of interest
While I can’t assert anything definitive, I can identify what I see as a severe conflict of interest underlying the entire CAEFISS system — one which presents one possibility as to why PHAC would have reason to be selective with which reports make it into the combined COVID-19 adverse event data, whether intentional or not.
Active surveillance for PHAC is administered by the Canadian Paediatric Society (CPS) through a separate partner program called the Canadian Immunization Monitoring Program ACTive, or IMPACT, which is “a paediatric hospital-based national active surveillance network for adverse events following immunization, vaccine failures and selected infectious diseases that are, or will be, vaccine preventable.”27 As shown in the CAEFISS graphic (shared again below), IMPACT is the source of hospital data for PHAC directly, but apparently also inserts reports at the level of the local public health units and federal/provincial/territorial health authorities.
In other words, this seems to suggest that hospitals in the IMPACT program have a certain level of discretion over how much scrutiny is applied to a given adverse event report. The earlier in the chain they go, the more red tape they come up against. It is entirely unclear to me what the purpose of this selective-input privilege held by IMPACT could be.
While PHAC is the primary funder for IMPACT, it is important to note that the program receives additional funding from GlaxoSmithKline, Merck, Novartis and Pfizer for “additional surveillance for rotavirus and invasive meningococcal disease.”28 Annual reports for the Canadian Paediatric Society from 2007 through 2012 further reveal that the organization has also received funding across multiple years from COVID-19 vaccine developers AstraZeneca, Janssen, Johnson & Johnson, Merck, and Sanofi Pasteur, as well as Pfizer, GSK and Novartis — and to add insult to injury, Purdue Pharma, the company largely blamed for kicking off the ongoing opioid crisis in North America.29303132
Many of these same companies have also sponsored one or more of CPS’ Annual Conferences, such as in 2019 and 2023:3334

The ongoing, current status of these relationships between CPS and the pharmaceutical companies is confirmed on page 11 of their Fall-Winter 2022 newsletter, well into the COVID-19 vaccine rollout:35

Beyond this direct conflict of interest, the Canadian Paediatric Society also received significant funding from the federal government to combat “vaccine hesitancy.” This included a $726,704 grant through the Public Health Agency of Canada’s Immunization Partnership Fund, intended to “increase vaccine confidence, uptake, and access to COVID-19 vaccines.”36 It’s a shame this amount of money wasn’t awarded to help CPS more accurately detect safety signals.

So, let’s get this straight: the CAEFISS system receives a significant portion of its adverse event reports from “hospital stakeholders” participating in the IMPACT program, which is funded in part by Pfizer — and led by an organization funded by AstraZeneca, Janssen and Pfizer, all of whom have great interest in not having their products be perceived as anything other than “safe and effective.”
Call me crazy, but this lay person perceives this as a conflict of interest.
In summary…
Canada has two health agencies, each with their own adverse event reporting system for vaccines.
Health Canada: Canada Vigilance Program (CVP)
Public Health Agency of Canada: Canadian Adverse Events Following Immunization Surveillance System (CAEFISS)
Canada decided it was a smart idea to deliver a selection of experimental, never-before-used gene therapy-based vaccine products with an unknown safety profile, despite the limited clinical trial data that did exist showing significant harm to recipients, presenting an unprecedented need to be ready and on the lookout for unexpected adverse events, to allow the products to be paused or pulled as necessary.
Just before the COVID-19 novel vaccine rollout began, Health Canada removed the ability for the vast majority of Canadians to report any adverse events they might suffer to the Canada Vigilance Program. This was based on the advice of a single, unnamed person/organization at the provincial level, who managed to change national pharmacovigilance policy with apparently remarkable ease.
Adverse event reports were largely redirected to PHAC’s CAEFISS, which forced people to go through their doctor/health care practitioner. Doctors were provided with absurdly narrow criteria for what constituted a “legitimate” adverse event, despite there existing no sufficient data from the Phase III clinical trials on which to rule out any adverse events. Doctors who reported adverse events of a quality or quantity outside of pre-determined bounds were systematically harassed, censured and removed from the medical system.
CAEFISS is fed in part by IMPACT, led by the Canadian Paediatric Society, which has close and long-term financial ties with the very pharmaceutical companies whose products they’re in charge of monitoring in the hospital setting.
Health Canada received multiple complaints from the general public and health care practitioners about this disastrous restriction on adverse event reporting.
After two years of thinking about it, Health Canada reintroduced the ability to report directly to their CVP — long after the COVID-19 vaccine campaign disappeared quietly into the background, and unthinkable numbers of people suffered illness, injury and death after taking these shots, the overwhelming majority of which likely went unreported.
Any questions?
As a postscript, I must note that this doesn’t account for the Vaccine Injury Support Program (VISP), a whole separate system set up in order to process claims of injury or death following receipt of a COVID-19 vaccine. I will save this for a future article so as to not overload you with information, but just understand that this already-complicated mess only gets even more absurd.
Thank you for reading, and I hope you found this informative! This is not the end of the story, and I thank you all for supporting me as paid subscribers to Microjourneys. This helps me continue my work independently and dedicate the necessary time to learn, understand, and communicate these important topics.
CBC News. (2020, April 9). “This is the new normal,” until COVID-19 vaccine developed: Trudeau. YouTube. https://web.archive.org/web/20221108025542/
Evidence - PACP (44-1) - No. 50. (2023, February 13). House of Commons of Canada. https://web.archive.org/web/20230626184844/https://www.ourcommons.ca/DocumentViewer/en/44-1/PACP/meeting-50/evidence
Ugolini, T. (2023, June 20). Health Canada says they’re reviewing Pfizer’s trickling release of safety data, but the timeline doesn’t add up. Rebel News. https://web.archive.org/web/20230626193052/https://www.rebelnews.com/health_canada_says_they_re_reviewing_pfizer_s_trickling_release_of_safety_data_but_the_timeline_doesn_t_add_up
Lamoureux, K. (2023, June 13). Q-1448 [Public Health Agency of Canada and Health Canada] by Mr. Carrie (Oshawa), April 25, 2023 = Q-1448 [Agence de la santé publique du Canada et Santé Canada] de M. Carrie (Oshawa), le 25 avril 2023. Library of Parliament. https://parl-gc.primo.exlibrisgroup.com/discovery/delivery/01CALP_INST:01CALP/12154760010002616
What is Pharmacovigilance? World Health Organization. Retrieved June 26, 2023, from https://web.archive.org/web/20230626190202/https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance
About Us. VAERS. Retrieved May 19, 2023, from http://archive.today/2023.05.19-122519/https://vaers.hhs.gov/about.html
McDonald, J. (2023, June 6). What VAERS Can and Can’t Do, and How Anti-Vaccination Groups Habitually Misuse Its Data. FactCheck.org. http://archive.today/2023.06.30-190001/https://www.factcheck.org/2023/06/scicheck-what-vaers-can-and-cant-do-and-how-anti-vaccination-groups-habitually-misuse-its-data/
Sunfellow On COVID-19. (2023, May 3). International Covid Summit III - Part 2 - European Parliament, Brussels. Rumble. https://rumble.com/v2nxmts-international-covid-summit-iii-part-2-european-parliament-brussels.html
Health Canada. (2017, August 8). Health Portfolio. Government of Canada. https://web.archive.org/web/20230327154718/https://www.canada.ca/en/health-canada/corporate/health-portfolio.html
Health Canada. (2023, March 23). Government of Canada. https://web.archive.org/web/20230626195056/https://www.canada.ca/en/health-canada.html
Hadju, P. (2020, September 17). ARCHIVED Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19. Government of Canada. http://archive.today/2021.02.26-180932/https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/interim-order-import-sale-advertising-drugs.html
Public Health Agency of Canada. (2020, December 12). Archived 1: Recommendations on the use of COVID-19 vaccine(s) [2020-12-12]. Government of Canada. https://web.archive.org/web/20230626200225/https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/december-12-2020.html
Health Canada. (2023, March 29). Summary Basis of Decision - Comirnaty (previously the Pfizer-BioNTech COVID-19 Vaccine). Government of Canada. https://covid-vaccine.canada.ca/info/summary-basis-decision-detailTwo.html?linkID=SBD00510
van Koeverden, A. (2023). Order Paper Question - Q-1448. Internet Archive; Government of Canada. https://archive.org/details/order-paper-question-q-1448
Health Canada. (2022, June 15). Canada Vigilance Program. Government of Canada. http://archive.today/2023.05.09-195237/https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/canada-vigilance-program.html
Health Canada. (2020, December 14). Adverse Reaction and Medical Device Problem Reporting. Government of Canada. http://archive.today/2021.08.14-122723/https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-reporting.html
Health Canada. (2022, September 1). Terms of use, privacy statement and interpretation of data to search the Canada vigilance adverse reaction online database. Government of Canada. https://web.archive.org/web/20230626205247/https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-database/medeffect-canada-caveat-privacy-statement-interpretation-data-search-canada-vigilance-adverse-reaction-online-database.html
Bernstein, S. (2010). Electronic Support for Public Health-Vaccine Adverse Event Reporting System (ESP:VAERS) (p. 6). Harvard Pilgrim Health Care. https://web.archive.org/web/20230630190016/https://digital.ahrq.gov/sites/default/files/docs/publication/r18hs017045-lazarus-final-report-2011.pdf
Public Health Agency of Canada. (2023, April 5). Canadian Adverse Events Following Immunization Surveillance System (CAEFISS). Government of Canada. https://web.archive.org/web/20230626205324/https://www.canada.ca/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html
Sturgess, L. (2023). A Citizens’ Hearing: Examining Canada’s Covid Response. Canadian Covid Care Alliance, 91–94. https://doi.org/10.31219/osf.io/sk3d5
Worldwide Safety Pfizer. (2021). 5.3.6 Cumulative analysis of post-authorization adverse event reports of PF-07302048 (BNT162B2) received through 28-FEB-2021. Food and Drug Administration. https://web.archive.org/web/20230629073047/https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf
Patrick Phillips [@DrP_MD]. (2021, May 5). “Unlike the transparent US VAERS system, PH depts in Canada are routinely rejecting reports of adverse events after vaccines, including serious ones that require hospitalization They only accept reports that meet strict arbitrary criteria This is a major risk to public safety”. [Tweet]. Twitter. https://web.archive.org/web/20210506090438/https://twitter.com/DrP_MD/status/1389892383941218307/photo/1
Dillman, M. (2023, June 7). Northern Ontario doctor’s licence revoked due to misconduct. CBC News. http://archive.today/2023.06.27-220850/https://www.cbc.ca/news/canada/sudbury/patrick-phillips-tribunal-college-1.6868061
Canadian Doctor Defies Gag Order and Tells the Public How the Moderna COVID Injections Killed and Permanently Disabled Indigenous People in His Community. (2021, April 26). Health Impact News. https://web.archive.org/web/20230628223414/https://assets-global.website-files.com/606d3dece4ec3c3866cc798a/60a601ccd6eac8d0fc8a311e_53%20Health%20Impact%20News%202021%20Canadian%20Doctor%20Defies%20Gag%20Order%20and%20Tells%20the%20Public.pdf
Fenton, C. (2021). Response Package HTH-2021-13807 (p. 20). Government of British Columbia. https://web.archive.org/web/20230628224056/http://docs.openinfo.gov.bc.ca/Response_Package_HTH-2021-13807.pdf
Public Health Agency of Canada. (2023, June 9). COVID-19 vaccine safety: Understanding the data on side effects following immunization. Government of Canada. https://web.archive.org/web/20230629200358/https://health-infobase.canada.ca/covid-19/vaccine-safety/utd.html
Surveillance. Canadian Paediatric Society. Retrieved March 28, 2022, from http://archive.today/2022.03.28-225952/https://cps.ca/en/impact
National Advisory Committee on Immunization (NACI): Membership and representation. (2020, December 18). Wayback Machine; Government of Canada. https://web.archive.org/web/20201218222110/https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/naci-membership-representation.html
Hilliard, R. I. (2011). Inside Stories: The Year in Review 2010-2011 (p. 21). Canadian Paediatric Society. https://web.archive.org/web/20230626213917/https://cps.ca/uploads/publications/AnnualReport-2010-2011-en.pdf
Pekeles, G. S. Outfront: Annual Report 2007-2008. Canadian Paediatric Society. Retrieved June 26, 2023, from https://web.archive.org/web/20230604214219/https://cps.ca/uploads/publications/AnnualReport07-08-1.pdf
Embree, J. E. Body, Mind, Spirit: Annual Report 2008-2009. Canadian Paediatric Society. Retrieved June 26, 2023, from https://web.archive.org/web/20230626221156/https://cps.ca/uploads/publications/AnnualReport08-09-1.pdf
Hirsch, R. (2017). The Opioid Epidemic: It’s Time to Place Blame Where It Belongs. Missouri Medicine, 114(2), 82–90. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6140023/
Canadian Paediatric Society 96th Annual Conference - Final Program. (2019). Canadian Paediatric Society. https://web.archive.org/web/20221125203653/https://annualconference.cps.ca/uploads/downloads/Final_Program_Toronto_2019.pdf
Annual Conference 2023 Program Guide. (2023). Canadian Paediatric Society. https://web.archive.org/web/20230626224104/https://cps.ca/uploads/annual_conference/halifax-program-2023_1.pdf
Brouillette, G., Moreau, E., Strickland, J., & Thistle, L. (2022). CPS News Fall-Winter 2022 (p. 11). Canadian Paediatric Society. https://web.archive.org/web/20230626223045/https://cps.ca/uploads/publications/CPSNews%28fall-winter22%29.pdf
Public Health Agency of Canada. (2022, October 12). Immunization Partnership Fund. Government of Canada. https://web.archive.org/web/20221104154209/https://www.canada.ca/en/public-health/services/immunization-vaccine-priorities/immunization-partnership-fund.html





















Very important breakdown. I thought I had seen a video recently that broke this down...
It's stuck in my head that you did it but I couldn't find it in a quick skim through microjourneys...
Either way, it's very hard to see this action as anything other than a demonstration of malicious intent.
The treatment of Patrick Phillips was especially grievous.
I appreciate the voice-over! Great read through!